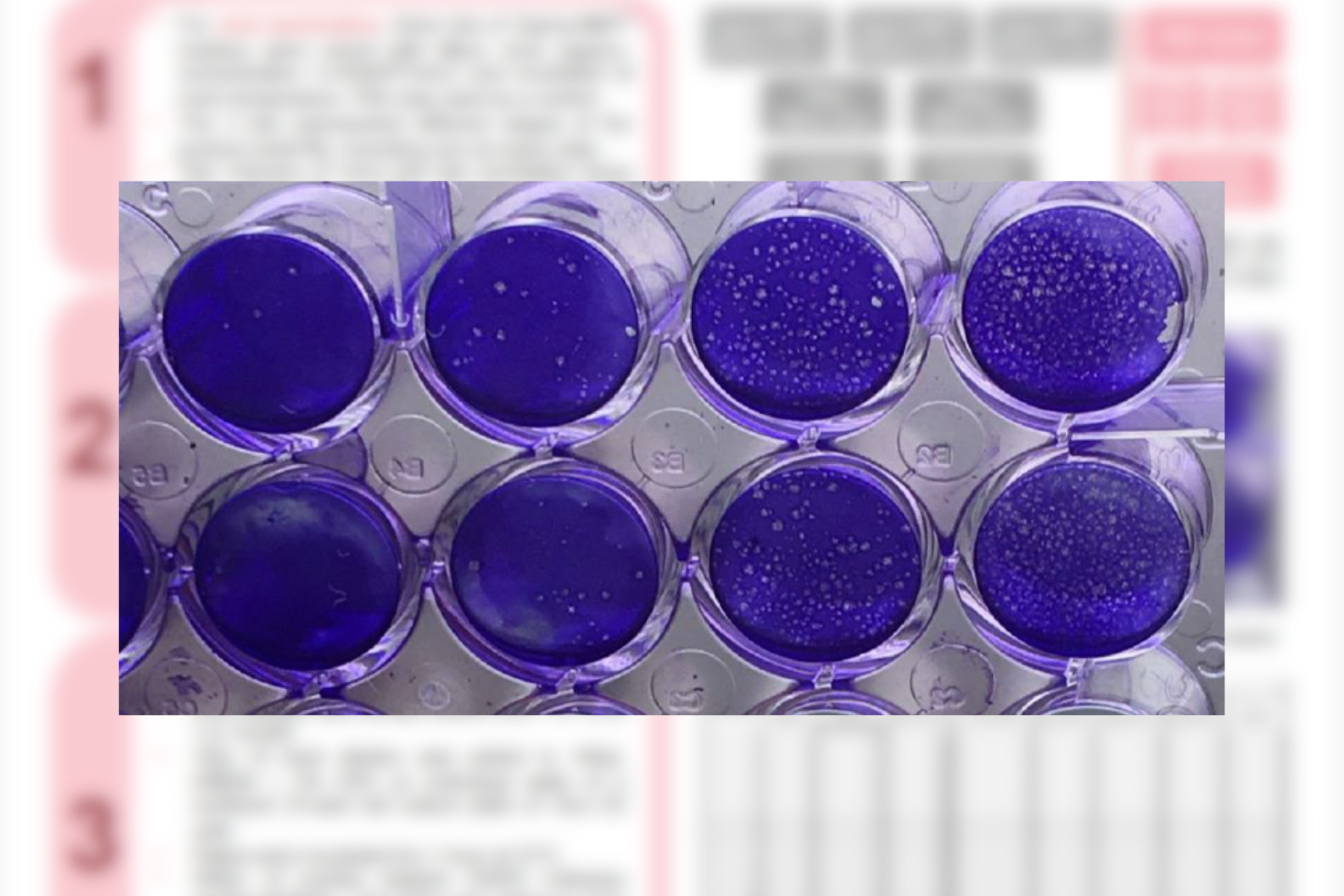
Poster presented at Minoritised Life Scientists Future Forum 2025 Tethi Biswas MWE, Corsham, United Kingdom Click for Full Poster: Evaluation of ENVIRO-KIT™, a new device for rapid detection of Listeria monocytogenes on food contact surfaces.
welcome to the MWE
KNOWLEDGE HUB
Our hub is here to provide you the most interesting and noteworthy goings on in our industry. Explore by browsing the most recent posts, or filter your search to find more specific articles. It’s updated regularly so don’t forget to keep returning for more. Enjoy!

Explore the Knowledge Hub
Filter by:
Currently filtering for:
All Knowledge Hub articles
Poster presented at 27th ESCV, Thessaloniki, Greece September 2025 Giasemi C. Eptaminitaki, Alexandros Zafiropoulos, George Sourvinos Laboratory of Clinical Virology, Medical School, University of Crete, Heraklion, Greece Click for Full Poster: Evaluation of SIGMA-MM™ Molecular Medium for the stability of SARS-CoV-2 virus for up to 90 days at different temperatures.
Poster presented at EMMD 13th European Meeting on Molecular Diagnostics, Noordwijk, October 09-11, 2024 Helen Jones & Jamran Khan, A1 Laboratory Services, LONDON, United Kingdom Click for Full Poster: Resilience in Action: Lessons Learned to Action Plans for the Next Global Pandemic
The need for safe specimen transportation and testing in areas with limited biological containment facilities has become increasingly urgent. SIGMA-MM™ has demonstrated effectiveness in inactivating various microorganisms, including RNA viruses like Influenza and SARS-CoV-2, while preserving nucleic acids for identification. In response to the Mpox outbreak in non-endemic countries in 2022, we aimed to test […]
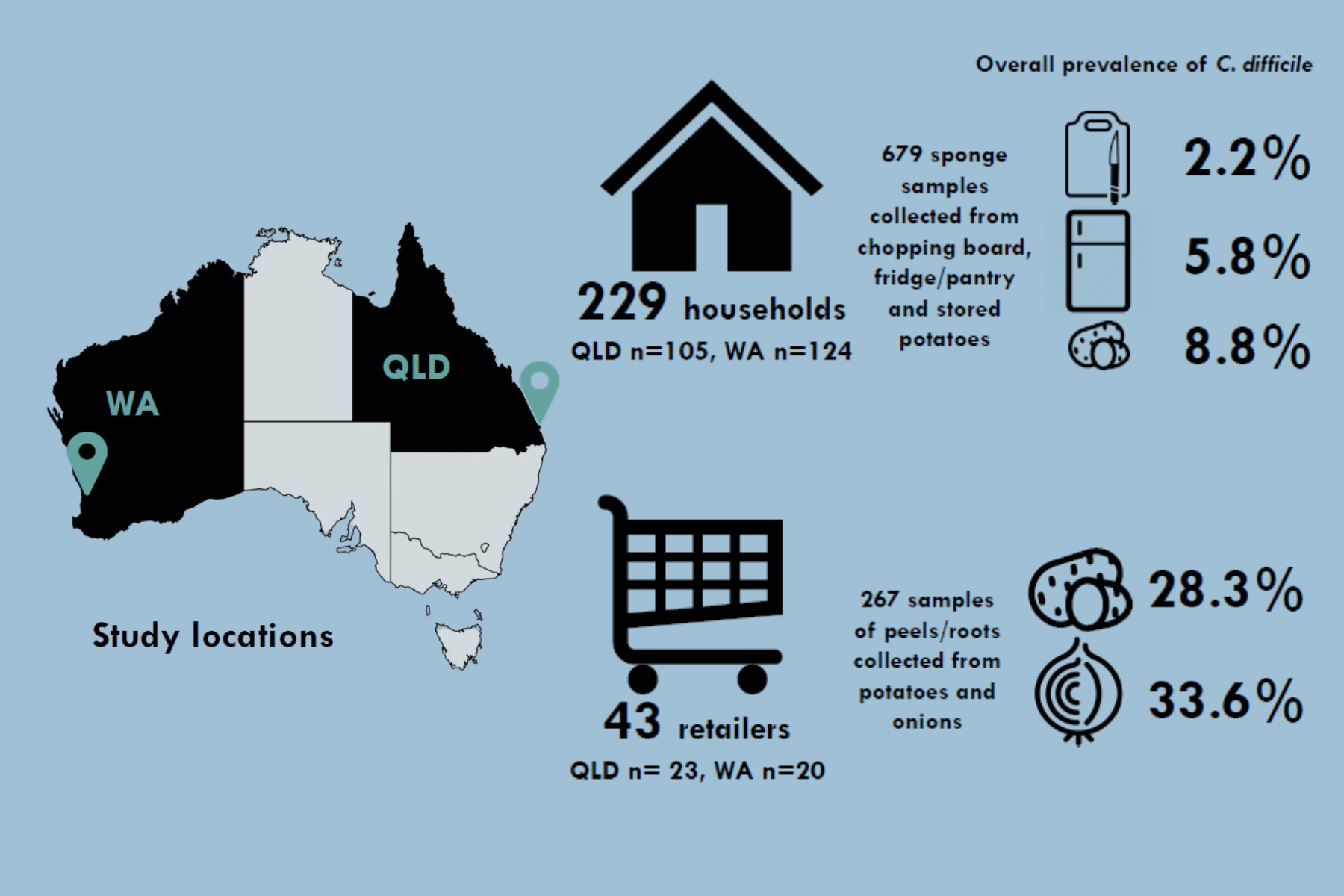
Community-associated Clostridium difficile infection (CA-CDI) has been rising in Australia for several years. Using MWE's POLYWIPE™, researchers from The University of Western Australia were able to determine the prevalence and molecular types of C. difficile present on reatil vegetables and households in Queensland and Western Australia
The recent reports of invasive Group A Streptococcal infections in children have drawn attention to this relatively common pathogen. MWE has a range of products that can help with diagnosis.
Earlier this year, former Chief Biomedical Scientist Ivor J Mitchelmore and his colleagues set up a microbiology culture service, where they used MWE swabs, media and Microloops to detect bacteria such as S. aureus, etc.
A study done by Cardiff University showed that SIGMA VCM™ transport medium met and exceeded the basic requirements for transport of bacteria set out by the Clinical and Laboratory Standards Institute Standard M40-A2. Viability of Ureaplasma species in SIGMA-VCM™ transport medium was stable for 4 days at room temperature. Viability could be extended to at least 264 hours if kept at 4˚C.
Recently, the World Health Organisation (WHO) released a report on acute hepatitis in children with an unknown origin. Even though Adenovirus is a hypothesis as being one of the strongly associated reasons for Hepatitis in this report, it is still under investigation. United Kingdom is one of the highly affected countries. PCR analysis has been the first line of diagnosis. Blood sampling, respiratory sampling and stool sampling are being used to collect specimen. Respiratory specimen in a VTM such as the SIGMA-VIROCULT® (Viral transport media) and SIGMA-MM™ inactivation media can be used to collect samples.
Poliovirus can be cultured from faeces which usually goes through a prolonged cell culture process. MWE’s FECAL TRANSWAB® compatible with PCR, would be a suitable medium to collect and transport the sample to the laboratory if faecal sample was not readily available or desirable.
Monkeypox virus webinar hosted by MWE and Prolab Diagnostics, featuring Neil Bentley, OBE, Mark Reed - Prolab Diagnostics, Richard Seuke - MWE
It is imperative that any sampling device does not carry non-viable (dead) organisms as they may guide users towards the wrong conclusion. MWE products consistently pass every aspect of M40-A2 requirements, including absence of non-viable organisms.
POLYWIPE™ sponges designed for collection of possible contaminants from a wide range of surfaces. These surfaces include production machinery in food factories, and carcasses in the butchering trade.
Great efforts are being made by healthcare professionals everywhere to reduce and if possible eliminate the risk of hospital acquired infections such as MRSA and Clostridium difficile. Nowhere is this more important than in the operating theatre, where every possible step is taken to ensure an environment that is as near sterile as possible to prevent surgical site infections.
The standard ISO 18593:2018 provides important guidance for sampling and testing surfaces in the food chain environment for microbiological safety, and much of this information is relevant for monitoring surfaces in healthcare settings for the purposes of infection control.
SIGMA-VCM™ proven to be reliable at storing and transporting Ureaplasma and Mycoplasma
SIGMA-GBS™ is a swab based device for the direct collection and rapid processing of screening specimens for Streptococcus agalactiae (commonly known as Group B Streptococcus).
SIGMA-SP™ contains a novel reagent which is not only a more powerful mucolytic, but comes in a simple, ready to use liquid format, ready to load onto the automated platforms.
MWE's VIABANK™ is a convenient, easy-to-use cryoprotection system for the storage of microorganisms
MWE’s Fecal transport swab means, you can now use your molecular systems to detect enteric pathogens in faecal specimens
SIGMA MM™ proven to inactivate SARS-CoV-2 in 60 seconds, preserving the RNA for safe transportation and testing
SIGMA MM™ is compatible with culture and molecular techniques
SIGMA MM™ inactivates deadly pathogens and preserving and maintaining DNA integrity
Collection and storage of viral specimen is the most important factor in obtaining a reliable and accurate diagnostic result.
CLSI’s ‘Quality Control of Microbiological Transport Systems’ provides a method to determine collection devices suitable for the transport of viral specimens over extended distances
SIGMA VIROCULT® has been proven to be effective at detection of Herpes on the BD Max™ and Smartcycler® automated platforms