Contact us
Phone
Address
Corsham, Wiltshire
England
SN13 9RT
Have a question or would like to find out about one of our products? Please fill in the form below and we’ll be in touch soon.
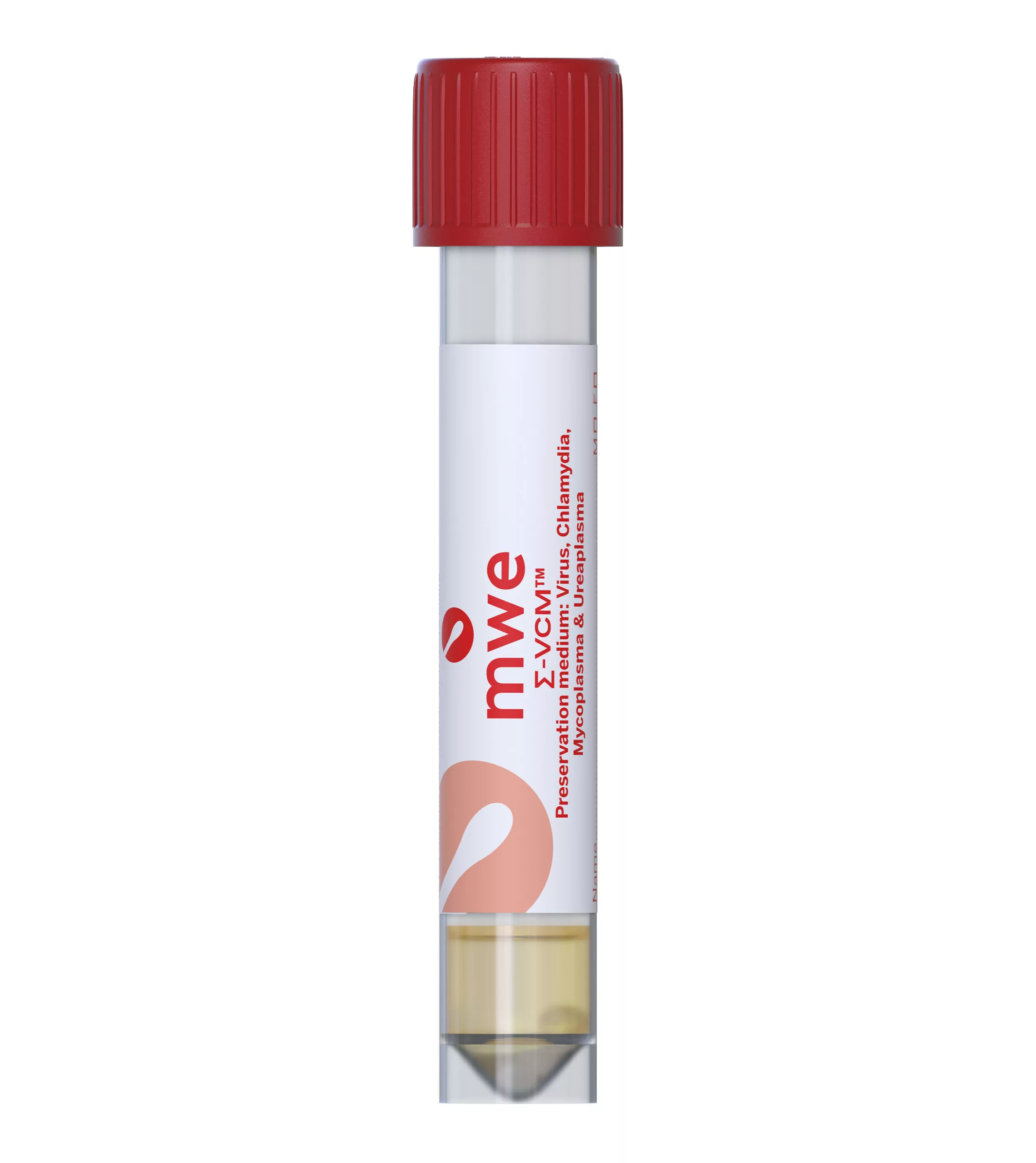
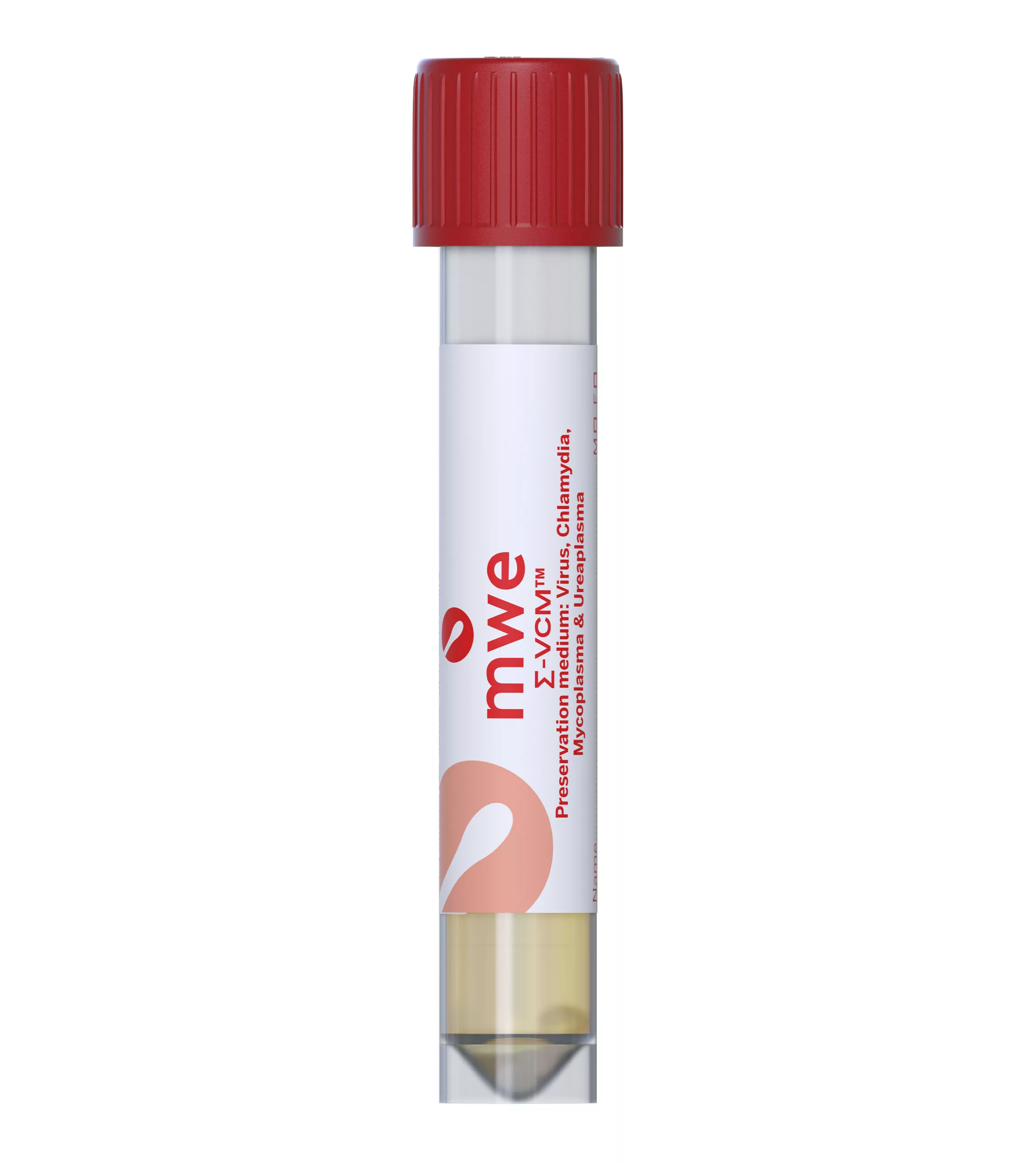
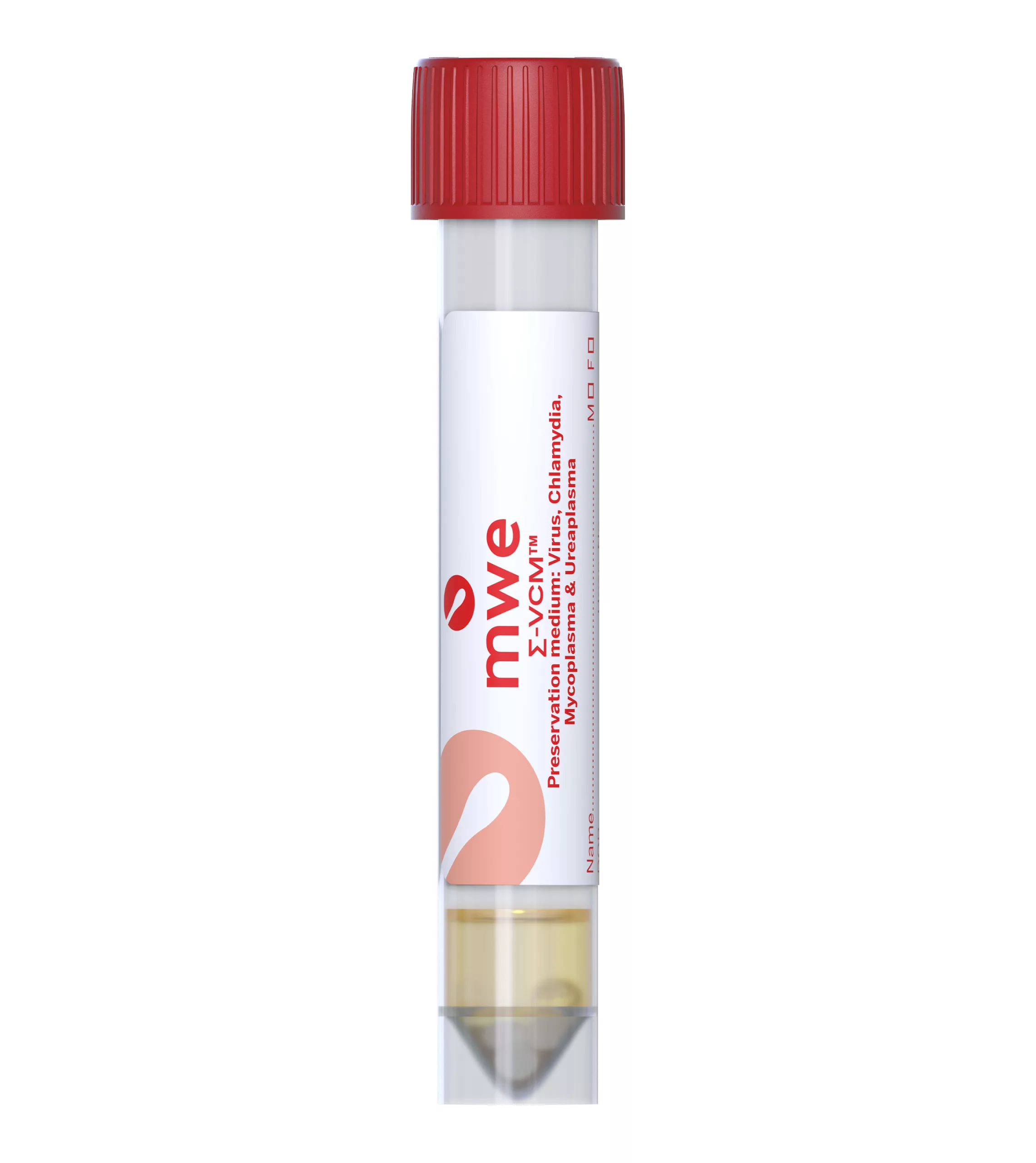
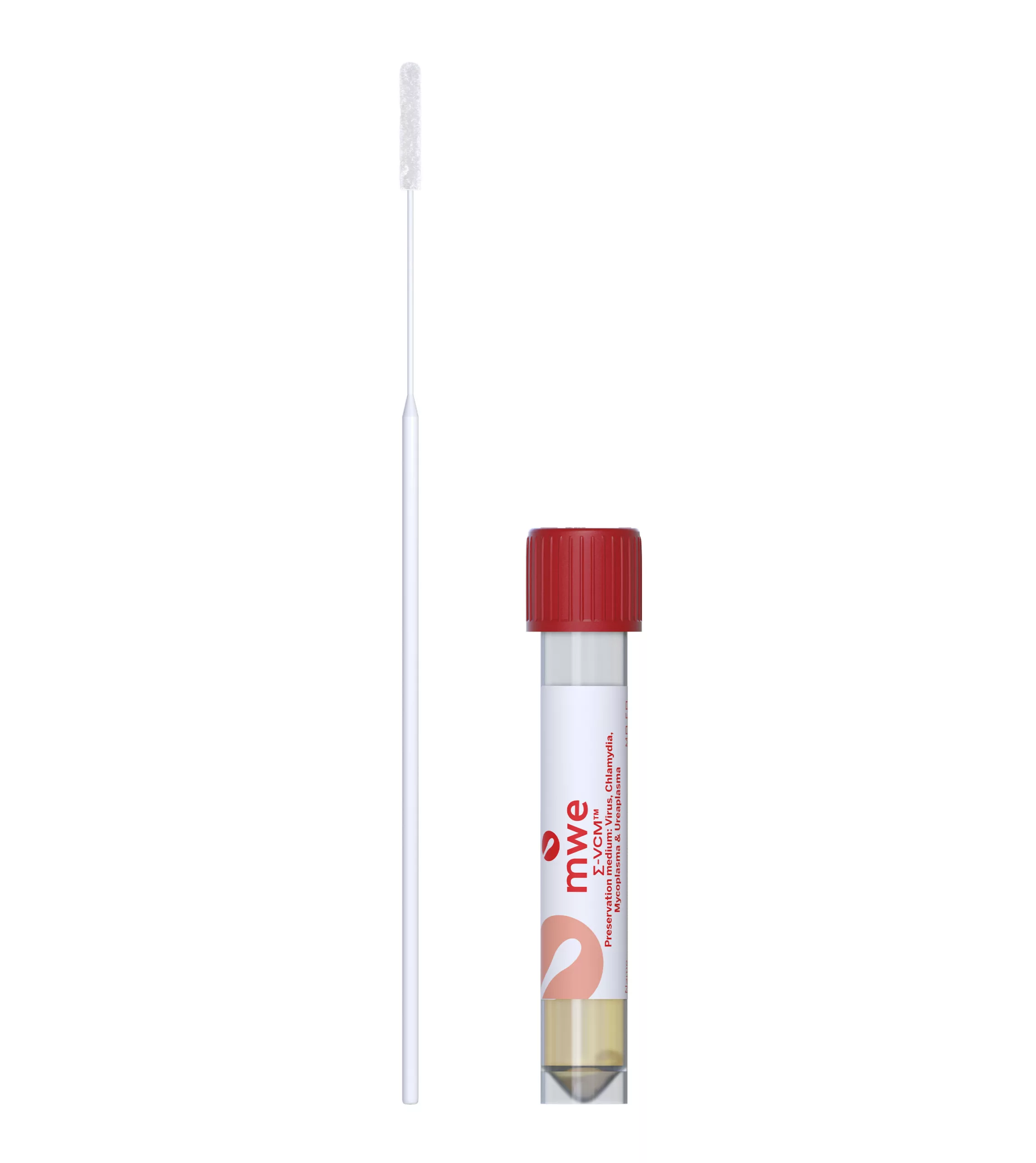
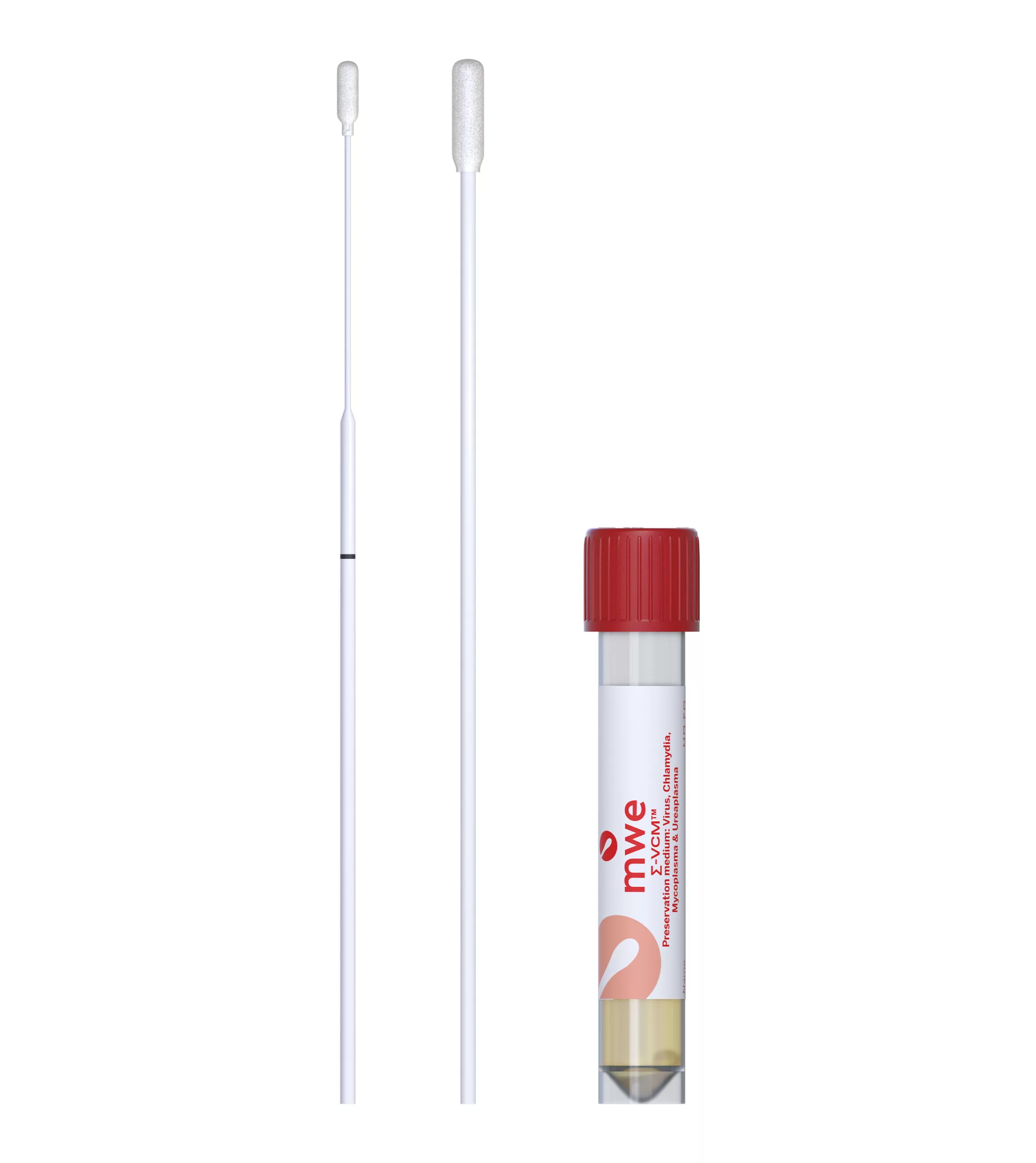
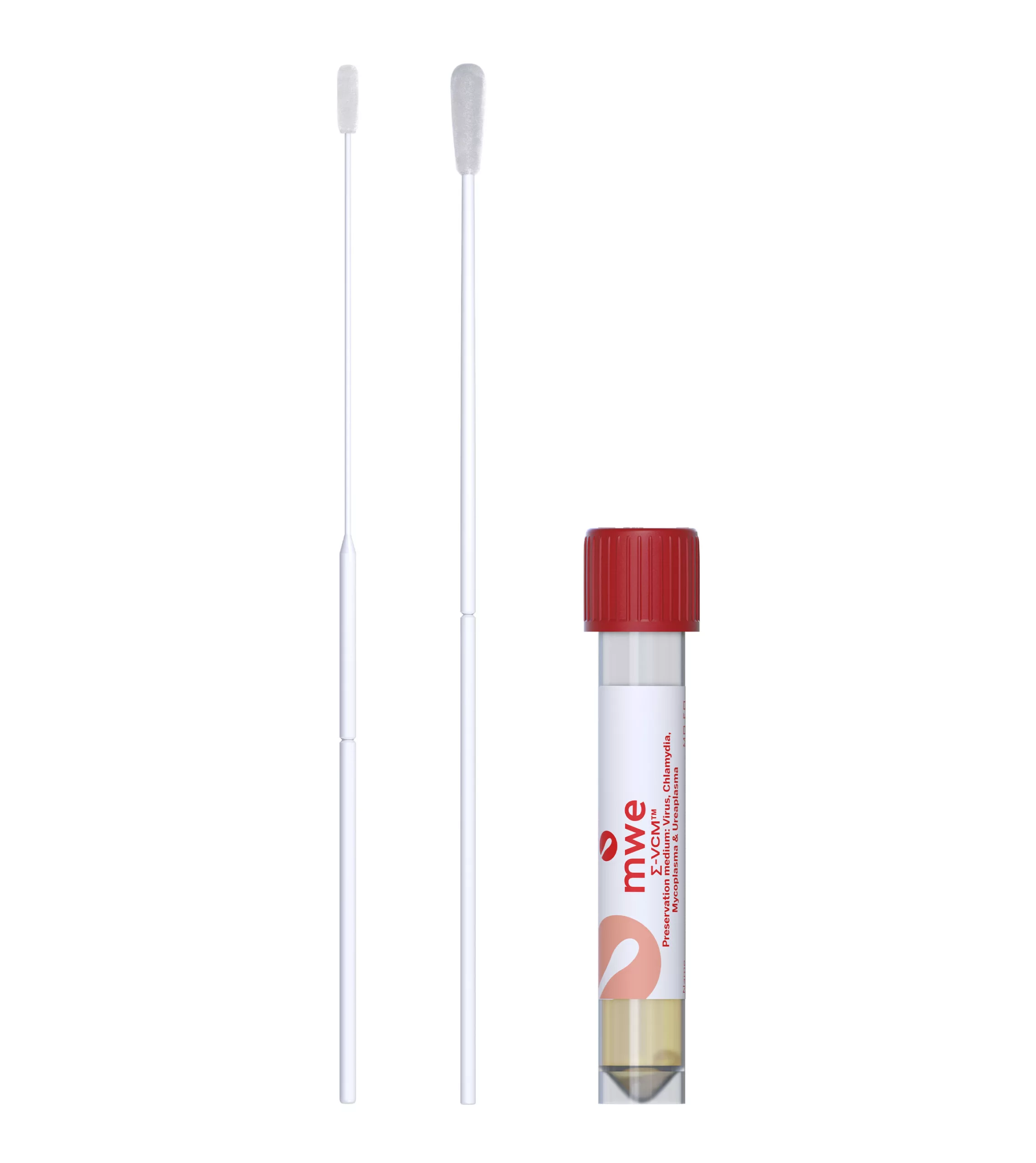
SIGMA-VCM™
SIGMA VCM™ is a preservation and a universal transport media device, used for optimum recovery of viruses, chlamydia, mycoplasma, ureaplasma and Neisseria Gonorrhoeae. It is compatible with culture and molecular diagnostic techniques.
Antibiotics in the medium inhibit the growth of contaminating bacteria and fungi. The medium can reduce the number of inappropriately taken samples, therefore avoiding the need to order repeat specimens. There is also no need for Sigma-VCM™ to be refrigerated or frozen for transport allowing lower cost ambient transportation.
Respiratory, skin and urogenital
- Sigma-VCM™ medium is a suitable for chlamydia and mycoplasmas, and also Neisseria gonorrheae. – A truly universal medium for sexually transmitted disease. The glass beads improve dispersion of specimen, and release of microorganisms from host cells resulting in a rapid response to testing.
- Antibiotics in the medium inhibit the growth of contaminating bacteria and fungi reducing the number of inappropriately taken samples, therefore avoiding the need to order repeat specimens.
- Suitable for molecular and conventional techniques including cell culture.
- Liquid phase allows complete dispersion of sample. Uniform suspension can be aliquoted for multiple test and specimens can be pooled. Ideal for epidemiology and surveillance studies.
- Ambient temperature storage – ideal for community-based surveillance and transport to reduce costs and complexity in transportation.
- Sigma-VCM™ designed for lower cost ambient transportation removing the need for higher refrigerated or frozen transport.
Small Vial – Tube size: 12mm (Ø) x 80mm (h) Cap size: 15mm (h) x 17mm (Ø) Tube with cap: 83mm (h)
Large Vial – Tube size: 16mm (Ø) x 100mm (h) x Cap size: 16mm (h) x 20mm (Ø) Tube with cap: 103mm (h)
| Product Code | Description | Cap Colour | Swab Length | Pack Size | Expiry (Months) |
|---|---|---|---|---|---|
| 1ML UNIVERSAL TRANSPORT MEDIA AND SWAB IN A PEEL POUCH | |||||
| MW910S | SIGMA VCM™ - Standard Plastic Foam Printed Breakpoint - 12x80 Vial - 1ml VCM™ Universal Medium | Red | 15cm | 125 | 12 |
| MW911S | SIGMA VCM™ - ENT Foam Swab Breakpoint - 12x80 Vial - 1ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW912S | SIGMA VCM™ - Standard + ENT Foam - 12x80 Vial - 1ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| M350101 | SIGMA VCM™ - Standard Plastic Flock Swab Printed Breakpoint - 12x80 Vial - 1ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| M350102 | SIGMA VCM™ - ENT Flock Swab Breakpoint - 12x80 Vial - 1ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| 2ML UNIVERSAL TRANSPORT MEDIA AND SWAB IN A PEEL POUCH | |||||
| MW910PF2ML | SIGMA VCM™ - Standard Flockswab Printed Breakpoint - 12x80 Vial - 2ml VCM™ Universal Medium | Red | 15cm | 125 | 12 |
| MW911PF2ML | SIGMA VCM™ - ENT Minitip Flockswab Printed Breakpoint - 12x80 Vial - 2ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW913PF2ML | SIGMA VCM™ - Ultrafine Flexible Minitip Flockswab Printed Breakpoint - 12x80 Vial - 2ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW914PF2ML | SIGMA VCM™ - Standard + ENT Minitip Flockswab Printed Breakpoint - 12x80 Vial - 2ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| 3ML UNIVERSAL TRANSPORT MEDIA AND SWAB IN A PEEL POUCH | |||||
| MW910S3ML | SIGMA VCM™ - Standard Plastic Foam Swab Printed Breakpoint - 12x80 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW911PF3ML | SIGMA VCM™ - ENT Minitip Flockswab Printed Breakpoint -12 x 80 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| M350103 | SIGMA VCM™ - Standard Plastic Flock Swab Printed Breakpoint - 12x80 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW918S | SIGMA VCM™ - Standard Plastic Foam Swab Printed Breakpoint - 16x100 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW919S | SIGMA VCM™ - ENT MinitipFoam Printed Breakpoint - 16x100 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW920S | SIGMA VCM™ - Standard Plastic + ENT Minitip Foam Printed Breakpoint - 16x100 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| MW921S | SIGMA VCM™ - 2 Standard Plastic Foam Printed Breakpoint - 16x100 Vial - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| M400010 | SIGMA VCM™ - Standard Plastic Flock Swab - 3ml VCM Universal Medium | Red | 15cm | 125 | 12 |
| TUBE ONLY UNIVERSAL TRANSPORT MEDIA | |||||
| MW915T | SIGMA VCM™ - 12x80 Vial - 1ml VCM Universal Medium | Red | N/a | 50 | 12 |
| MW916T | SIGMA VCM™ - 12x80 Vial - 3ml VCM Universal Medium | Red | N/a | 50 | 12 |
| MW926T | SIGMA VCM™ - 16x100 Vial - 3ml VCM Universal Medium | Red | N/a | 50 | 12 |
Contact us
Phone
Address
Corsham, Wiltshire
England
SN13 9RT
Have a question or would like to find out about one of our products? Please fill in the form below and we’ll be in touch soon.
Product enquiry
For buying enquiries, or for more information about this product, please fill in the form below and we’ll be in touch soon.
Let’s see...
We can direct you to the best place. Would you like help with: